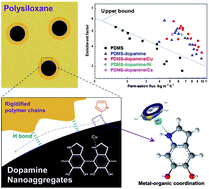
In this study, a series of hybrid membranes with high separation performance and superior swelling-resistance were fabricated by incorporating metal ion-chelated dopamine nanoaggregates into a poly(dimethylsiloxane) (PDMS) bulk matrix membrane. The concomitant hydrogen bond, metal-organic coordination and π-complexation interactions render the synergy of a favorable free volume property, reinforced chain rigidity and facilitated transport function within the membranes. The membranes displayed simultaneously enhanced permeation flux and enrichment factors when utilized for model gasoline separation. Especially, when the weight fraction of dopamine/Cu reached 5.0 wt%, the membrane displayed an optimum separation performance with a permeation flux of 7.42 kg m−2 h−1 (2.7 times as much as that of the PDMS control membrane) and an enrichment factor of 4.81 (11% more than that of the PDMS control membrane). Thanks to the elevated cohesive energy and the chain extension effect, the swelling degree of the PDMS-dopamine/Cu membranes decreased remarkably with the dopamine/Cu content. This study may provide a novel route to the design and fabrication of robust, high-performance hybrid membranes to meet diverse energy and environment-related application requirements.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...