Retracted Article: Monodisperse Ni3Fe single-crystalline nanospheres as a highly efficient catalyst for the complete conversion of hydrous hydrazine to hydrogen at room temperature†
Abstract
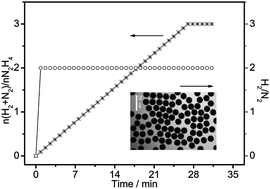
A facile, reliable, and robust synthetic method for the preparation of monodisperse Ni3Fe single-crystalline nanospheres with 182.3 m2 g−1 specific surface area is reported. The results of an investigation of the shape/size distribution, structural characteristics, and composition of these Ni3Fe nanospheres are presented, and a possible mechanism for the formation of the nanospheres at high temperatures in a cetylamine–palmitic acid solution in the presence of tri-iso-butyl thallium and borane trimethylamine complexes is proposed. During the decomposition of

 Please wait while we load your content...
Please wait while we load your content...