Tuning the amino acid sequence of minimalist peptides to present biological signals via charge neutralised self assembly†
Abstract
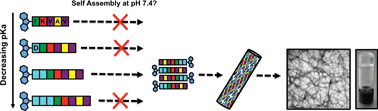
Nanofibrous materials yielded by the self-assembly of peptides are rich in potential; particularly for the formation of scaffolds that mimic the landscape of the host environment of the cell. Here, we report a novel methodology to direct the formation of supramolecular structures presenting desirable amino acid sequences by the self-assembly of minimalist peptides which cannot otherwise yield the desired scaffold structures under biologically relevant conditions. Through the rational modification of the pKa, we were able to optimise ordered charge neutralised assembly towards in vivo conditions.
 Please wait while we load your content...
Please wait while we load your content...