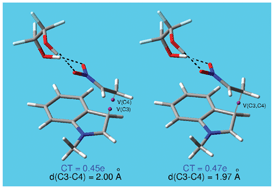
The Friedel–Crafts (FC) reaction of N-methyl indole 1 with nitroethylene 2 has been studied using DFT methods at the B3LYP/6-31+G** level in order to characterize the bonding changes along the C–C bond-formation process in polar reactions. For this FC reaction a two-step mechanism has been found. The first step is associated with the C–C bond formation between the most electrophilic centre of nitroethylene and the most nucleophilic centre of N-methyl indole, to yield a zwitterionic intermediate IN. The second step corresponds to an intramolecular proton transfer process at IN, regenerating the aromatic system present at the indole. Despite the high electrophilic character shown by nitroethylene 2, the electrophilic attack is not favoured, and the FC reaction is experimentally catalyzed by a bisamide. ELF bonding analysis along the first step provides a complete characterization of the electron density changes along the C–C bond formation, which begins at a distance of 1.97 Å by a C-to-C coupling of two pseudoradical centres located at the most electrophilic centre of nitroethylene complex 10 and the most nucleophilic centre of N-methyl indole 1. Our proposed reactivity model for the C–C bond formation in polar processes is completely different to that proposed by the FMO theory, in which the HOMO electrons of the nucleophile must interact with the LUMO of the electrophile.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...