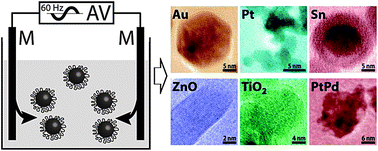
A simple and generic approach—alternating voltage induced electrochemical synthesis (AVIES)—has been reported for synthesizing highly dispersed colloidal metal (Au, Pt, Sn, and Pt–Pd) and metal oxide (ZnO and TiO2) nanocrystals. The respective nanocrystals are produced when a zero-offset alternating voltage at 60 Hz is applied to a pair of identical metal wires, which are inserted in an electrolyte solution containing capping ligands. In the case of Au, the obtained nanocrystals are highly crystalline nano-icosahedra of 14 ± 2 nm in diameter, the smallest Au icosahedra synthesized in aqueous solutions via green chemistry. Their catalytic activity has been demonstrated through facilitating the reduction of 4-nitrophenol to 4-aminophenol by sodium borohydride. This AVIES approach is an environmentally benign process and can be adopted by any research lab.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Please wait while we load your content...
Please wait while we load your content...