Dimethylformamide-mediated synthesis of water-soluble platinum nanodendrites for ethanoloxidation electrocatalysis†
Abstract
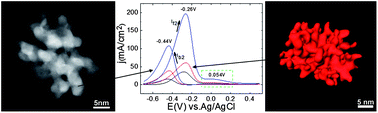
Herein we describe the synthesis of water-soluble platinum nanodendrites in
* Corresponding authors
a
Departamento de Quimica Fisica, Universidade de Vigo, 36310 Vigo, Spain
E-mail:
mourdik@uvigo.es, llizmarzan@cicbiomagune.es
b CIQ-UP L4, Faculdade de Ciencias, Universidade do Porto, Rua do Campo Alegre, 687, 4169-007 Porto, Portugal
c EMAT, University of Antwerp, Groenenborgerlaan 171, B-2020 Antwerp, Belgium
d BioNanoPlasmonics Laboratory, CIC biomaGUNE, Paseo de Miramón 182, 20009 San Sebastián, Spain
e Ikerbasque, Basque Foundation for Science, 48011 Bilbao, Spain
Herein we describe the synthesis of water-soluble platinum nanodendrites in

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
S. Mourdikoudis, M. Chirea, T. Altantzis, I. Pastoriza-Santos, J. Pérez-Juste, F. Silva, S. Bals and L. M. Liz-Marzán, Nanoscale, 2013, 5, 4776 DOI: 10.1039/C3NR00924F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
