Synthesis, photophysics and electrochemistry of novel, nitrogen-containing heterocyclic derivatives†
Abstract
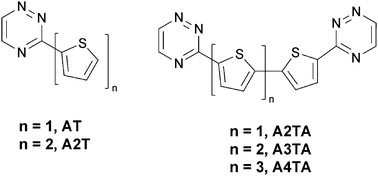
A series of new π-conjugated donor (
* Corresponding authors
a
Siedlce University, Institute of Chemistry, 3 Maja 54, 08-110 Siedlce, Poland
E-mail:
dankab@uph.edu.pl
Fax: +48 25 643 1093
Tel: +48 25 643 1128
b Silesian University of Technology, Faculty of Chemistry, Strzody 9, 44-100 Gliwice, Poland
c Centre of Polymer and Carbon Materials, Polish Academy of Sciences, M. Curie-Skłodowskiej 34, 41-819 Zabrze, Poland
d Institute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
e University of Silesia, Institute of Chemistry, Faculty of Mathematics, Physics and Chemistry, Szkolna 9, 40-007 Katowice, Poland
A series of new π-conjugated donor (

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
D. Branowska, B. Chaciak, O. Siuchta, E. Olender, P. Ledwon, M. Lapkowski, E. Poronik, W. Wysocki, Z. Karczmarzyk, L. Skora, M. Filapek, S. Krompiec, Z. Urbanczyk-Lipkowska and P. Kalicki, New J. Chem., 2013, 37, 1982 DOI: 10.1039/C3NJ00078H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
