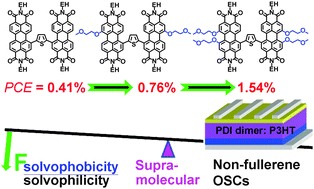
Because of their outstanding molecular optoelectronic properties, perylene diimides (PDIs) are promising alternatives to the commonly used PCBM. However, the overly strong aggregation ability, poor solution-processability and compatibility of PDIs severely limit their photovoltaic applications. We turned to borrowing the amphiphile concept to improve these supramolecular properties. Practically, we fine-tuned the molecular solvophobicity with respect to the molecular solvophilicity, e.g. Fsolvophob/solvophil, by changing the number of the weakly solvophobic 2-methoxyethoxyl (EG) groups in the bay-region of the thienyl-bridged dimeric PDI backbone, forming three PDI dimers of Bis-PDI-T (0 EG), Bis-PDI-T-EG (2 EG) and Bis-PDI-T-di-EG (4 EG) (Scheme 1). The photovoltaic properties using these dimers as the solution-processed non-fullerene electron-acceptor and P3HT as the electron-donor were investigated via the device configuration of ITO/PEDOT:PSS/P3HT:PDI dimer/Ca/Al. Bis-PDI-T exhibited overly strong aggregation ability and very poor solution-processability, which severely limited compatibility, giving a very poor power conversion efficiency (PCE) of 0.007%. When two EG groups were attached at the 1,1′-positions, the resulted Bis-PDI-T-EG showed dramatically reduced aggregation ability, improved solution-processability, compatibility and proper phase separation. Small sized phases (∼20 nm) dominated in the active layer and the best PCE was increased to 0.39%. When four solvophobic EG functions were introduced, affording Bis-PDI-T-di-EG with excellent supramolecular properties, particularly, the improvement of the phase separation with an increased phase size of 24 nm and the enhanced electron and hole mobilities, by 2–4 times, with respect to that of Bis-PDI-T-EG. The best PCE was further enhanced to 0.88%. After using 1-chloronaphthalene as the co-solvent of 1,2-dichlorobenzene to further improve the compatibility, the PCE was improved further up to 0.41% for Bis-PDI-T, 0.76% for Bis-PDI-T-EG and 1.54% for Bis-PDI-T-di-EG.

 Please wait while we load your content...
Please wait while we load your content...