Membrane disordering is not sufficient for membrane permeabilization by islet amyloid polypeptide: studies of IAPP(20–29) fragments
Abstract
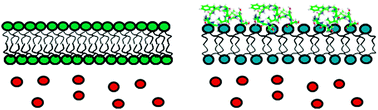
A key factor in the development of type II diabetes is the loss of insulin-producing beta-cells. Human islet amyloid
- This article is part of the themed collection: Biophysical studies on protein misfolding and amyloid diseases

 Please wait while we load your content...
Please wait while we load your content...