Do inverse dithienylethenes behave as normal ones? A joint spectroscopic and theoretical investigation†
Abstract
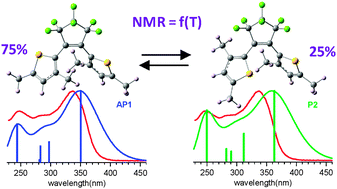
We investigate an inverse (I)
* Corresponding authors
a
Laboratoire de Spectrochimie Infrarouge et Raman (UMR 8516 du CNRS), Université des Sciences et Technologies de Lille, Bat C5, 59655 Villeneuve d'Ascq Cedex, France
E-mail:
stephane.aloise@univ-lille1.fr
b UDSL, CNRS UMR 8516, Univ Lille Nord de France, Lille, France
c Univiversité Paris 7 Denis Diderot, Sorbonne Paris Cité, ITODYS, UMR 7086, 15 rue Jean-Antoine de Baïf, 75205 Paris Cedex 13, France
d Université de Nantes, UFR des Sciences et des Techniques, UMR CNRS 6230, CEISAM, 2 rue de la Houssinière, 44322 Nantes Cedex 3, France and Institut Universitaire de France, 103 blvd St Michel, 75005 Paris Cedex 5, France
e Department of Chemistry and Applied Chemistry, Faculty of Science and Engineering, Saga University, Honjo 1, Saga 840-8502, Japan
We investigate an inverse (I)

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
S. Aloïse, M. Sliwa, G. Buntinx, S. Delbaere, A. Perrier, F. Maurel, D. Jacquemin and M. Takeshita, Phys. Chem. Chem. Phys., 2013, 15, 6226 DOI: 10.1039/C3CP43806F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
