Pressure dissociation of β-lactoglobulin oligomers near their isoelectric point
Abstract
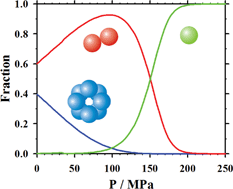
We study pressure dissociation of aggregated states of β-lactoglobulin at pH 4.6 using static and dynamic light scattering. We find that octamers dissociate at 20 °C into dimers at pressures P < 100 MPa and into monomers at P < 200 MPa. The dimer–monomer dissociation equilibrium at T = 20 °C is reversible. The dodecamer–dimer equilibrium is quasi-reversible since aggregation occurred after decompression from 30 MPa back to ambient pressure. The corresponding molar volume difference is ΔV = 101 ml mol−1 for the octamer–dimer and ΔV = 276 ml mol−1 for the dimer–monomer transition. The calculated free energy of association for the dimers at atmospheric pressure is ΔG = −59.3 kJ mol−1 and for the monomers is ΔG = −59.8 kJ mol−1. The high pressure dissociation is not emphasized by lowering the temperature. Instead, the quaternary structure of β-lactoglobulin at T = 10 °C remains unchanged up to pressures of P = 180 MPa followed by aggregation at pressures P > 180 MPa.
- This article is part of the themed collection: International Symposium on Food Rheology and Structure

 Please wait while we load your content...
Please wait while we load your content...