Self-assembly and luminescence of pyrazole supergelators†
Abstract
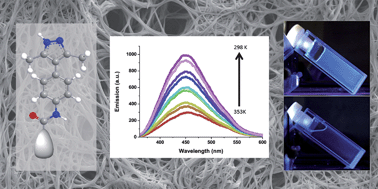
Functional supramolecular organogelators containing a luminescent 4-arylpyrazole unit are described. Moreover, the compounds show supergelator behaviour. The synthesised molecules combine a 1H-pyrazole ring, an amide group and a trialkoxyphenyl group. The study of the gelation process and the structure–property relationship reveals that both the

 Please wait while we load your content...
Please wait while we load your content...