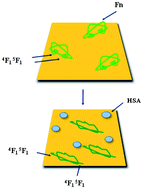
The effect of coadsorption on the conformational arrangement of human plasma fibronectin (Fn) was studied for mixtures with human serum albumin (HSA) adsorbed onto mildly hydrophilic gold substrates. Quartz crystal microbalance with dissipation monitoring (QCM-D) and atomic force microscopy (AFM) were used to measure the mass uptake, thickness, viscoelastic behaviour, and morphology of the adsorbed protein adlayers. Time-of-flight secondary ion mass spectrometry (ToF-SIMS) was employed to determine the composition of binary protein adlayers, taking advantage of the principal component analysis (PCA) technique of ToF-SIMS data. Thus, the ToF-SIMS results provided the particular fragmentation patterns of the two proteins, showing that the resulting mixed protein layers were predominantly formed by Fn molecules, even for binary solutions with high molar fraction of HSA. The conformational arrangement of the Fn molecules was studied by combining ToF-SIMS and QCM-D techniques. ToF-SIMS data allowed the identification of Type I–Type III modules of Fn and showed that pure Fn layers predominantly expose Type III modules, while coadsorbed Fn/HSA layers predominantly expose Fn Type I epitopes. QCM-D was employed to measure the relative uptake of a polyclonal antibody (anti-Fn) to the 4F15F1 binding domain in the Fn Hep I fragment in Type I modules, showing that pure Fn adlayers have a reduced anti-Fn binding capacity, as expected for Type I modules buried within the adlayers, while coadsorbed Fn layers bind more efficiently the anti-Fn, as the concerned Type I module is predominantly exposed at the layer surface. The results overall demonstrated that coadsorption of Fn and HSA onto mildly hydrophilic gold substrates prompts Fn to undergo a closed-to-open conformational switch.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...