Total synthesis of ageliferin via acyl N-amidinyliminium ion rearrangement†
Abstract
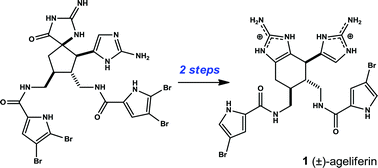
Ageliferin is a marine natural product having antiviral and antimicrobial activities. These functions remain to be characterized at a molecular level. Ageliferin is also thought a biosynthetic intermediary linking oroidin type

 Please wait while we load your content...
Please wait while we load your content...