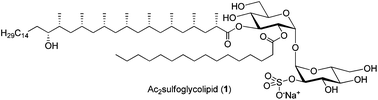
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), continues to represent a challenging pathogen causing many deaths. A reason for the persistence of this pathogen is the cell-envelope composition, which consists of long-tailed (glyco)lipids, involved in the modulation of the host immune response. Diacylated sulfoglycolipid Ac2SGL (1), found in the cell envelope, is a potent antigen that stimulates the immune response towards TB. This observation suggests the application of 1 as part of a vaccine. Here, we report the first asymmetric total synthesis of 1. Two approaches were developed for the synthesis of hydroxyphthioceranic acid (4), its polypropionate part, thereby establishing the absolute stereochemistry of the C17 hydroxyl function to be of (R)-configuration. Subsequently, 4 was regioselectively connected to the trehalose core and after selective sulfation and a final fourfold deprotection step, pure 1 was obtained. The identity of synthetic and natural 1 was confirmed by NMR and mass analysis, Furthermore, synthetic 1 shows identical biological function to 1 and activates CD1b-restricted and Ac2SGL-specific T cells that are highly sensitive to minimal structural modifications of 1 with the same potency. A modeling study is presented to point out the structural features of 1 that are important for binding to the antigen-presenting molecule CD1b and to the T-cell receptor.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...