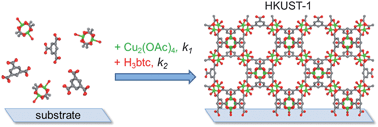
We describe a systematic investigation of the factors controlling step-by-step growth of the metal–organic framework (MOF) [Cu3(btc)2(H2O)3]·xH2O (also known as HKUST-1), using quartz crystal microbalance (QCM) electrodes as an in situ probe of the reaction kinetics and mechanism. Electrodes coated with silica, alumina and gold functionalized with OH– and COOH–terminated self-assembled monolayers (SAMs) were employed to determine the effects of surface properties on nucleation. Deposition rates were measured using the high sensitivity available from QCM-D (D = dissipation) techniques to determine rate constants in the early stage of the process. Films were characterized using grazing incidence XRD, SEM, AFM, profilometry and reflection–absorption IR spectroscopy. The effects of reaction time, concentration, temperature and substrate on the deposition rates, film crystallinity and surface morphology were evaluated. The initial growth step, in which the surface is exposed to copper ions (in the form of an ethanolic solution of copper(II) acetate) is fast and independent of temperature, after which all subsequent steps are thermally activated over the temperature range 22–62 °C. Using these data, we propose a kinetic model for the Cu3(btc)2 growth on surfaces that includes rate constants for the individual steps. The magnitude of the activation energies, in particular the large entropy decrease, suggests an associative reaction with a tight transition state. The measured activation energies for the step-by-step MOF growth are an order of magnitude lower than the value previously reported for bulk Cu3(btc)2 crystals. Finally, the results of this investigation demonstrate that the QCM method is a powerful tool for quantitative, in situ monitoring of MOF growth in real time.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...