Thermally stable N2 and H2 adducts of cationic nickel(ii)†
Abstract
The first examples of thermally stable molecular dihydrogen adducts of nickel were synthesized from their dinitrogen adduct precursors, which are themselves among the first examples of Ni(II)-N2 complexes. The minimal activation of the bound N2 moieties suggests that these adducts are stabilized predominantly through σ-donation from the adduct to the electrophilic metal center. We further show that the bound H2 ligand can undergo heterolytic cleavage to deliver hydride to the nickel center. The H2 adducts are of particular interest in the context of hypotheses suggesting that Ni can serve as the site for H2 binding and heterolytic activation in [NiFe] hydrogenases.
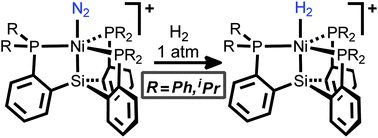

 Please wait while we load your content...
Please wait while we load your content...