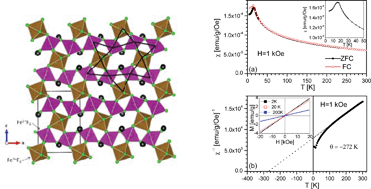
A new charge-ordered magnetically frustrated mixed-metal fluoride with a pyrochlore-related structure has been synthesized and characterized. The material, RbFe2F6 (RbFe2+Fe3+F6) was synthesized through mild hydrothermal conditions. The material exhibits a three-dimensional pyrochlore-related structure consisting of corner-shared Fe2+F6 and Fe3+F6 octahedra. In addition to single-crystal diffraction data, neutron powder diffraction and magnetometry measurements were carried out. Magnetic data clearly reveal strong antiferromagnetic interactions (a Curie–Weiss temperature of −270 K) but sufficient frustration to prevent ordering until 16 K. No structural phase transformation is detected from the variable-temperature neutron diffraction data. Infrared, UV-vis, thermogravimetric, and differential thermal analysis measurements were also performed. First-principles density functional theory (DFT) electronic structure calculations were also done. Crystal data: RbFe2F6, orthorhombic, space groupPnma (no. 62), a = 7.0177(6), b = 7.4499(6), c = 10.1765(8) Å, V = 532.04(8) Å3, Z = 4.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...