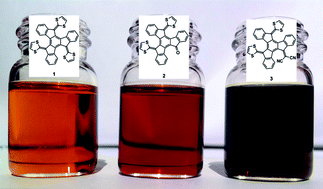
We describe the synthesis, electronic, optical and photophysical properties of a family of three electron-donor bowl-shaped organic molecules that absorb light in the whole range of the visible spectrum (up to 800 nm in one case), and associate C60 in solution with binding constants in the range of 104–102 M−1 as measured from both UV-vis and fluorescence titrations in several solvents. These molecules are π-extended derivatives of tetrathiafulvalene, based on a truxene core to which two or three units of dithiole are covalently attached. The inclusion of the bulky dithiole groups is responsible for their bowl-shape geometry, which allows them to associate with C60, and their electron-donor character. The symmetric derivative 1, with three dithiole units, absorbs light in the 370–520 nm range. Exchanging one of the dithiole groups by an electron-withdrawing group, ketone (2) and dicyanomethylene (3), results in an intramolecular push–pull effect that extends the absorption to nearly 700 nm in the case of 2, and up to 800 nm in the case of 3. Transient absorption measurements, supported by spectroelectrochemical and radiolytical experiments, reveal that upon photoexcitation of the 1∙C60 associate the fully charge-separated state 1•+∙C60•− is generated, with lifetimes of hundreds of picoseconds. Molecular-level understanding of the electronic and supramolecular properties of 1–3 is provided by density functional theory calculations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...