Unexpected radical polymerization behavior of oligo(2-ethyl-2-oxazoline) macromonomers†
Abstract
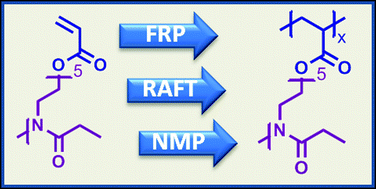
A well-defined oligo(2-ethyl-2-oxazoline)acrylate (OEtOxA) macromonomer was obtained by direct end functionalization of the living cationic oxazolinium species from the cationic ring-opening polymerization of EtOx with in situ deprotonated acrylic acid. Kinetic studies during subsequent reversible addition–fragmentation chain transfer (RAFT)

 Please wait while we load your content...
Please wait while we load your content...