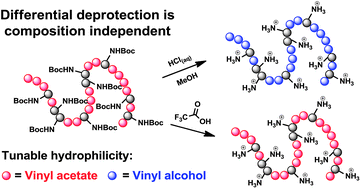
Toward the goal of developing degradable polycationic materials with tunable backbone charge densities and hydrophilicities, we report the optimized syntheses and polymerization activities of a series of N-Boc-protected amino acidO-vinyl ester (BAAVE) monomers derived from Boc-protected glycine, alanine, valine, and proline. The homopolymerization and copolymerization behaviors of these monomers under thermally initiated conventional free radical polymerization conditions are studied. By conducting copolymerizations of (N-tert-butoxycarbonyl)glycine vinyl ester (BGVE) with vinyl acetate (VAc) at various feed ratios and by analyzing the compositions of the resulting polymers produced at 88 °C using 1,1′-azobis(cyclohexane-1-carbonitrile) (V-40) initiation, we find that the reactivity ratios are rBGVE = 1.61 ± 0.12 and rVAc = 0.82 ± 0.07. Treatment of poly(VAc-co-BAAVE) with neat CF3COOH selectively unmasks the Boc-protected amine functionalities to furnish cationic poly(VAc-co-AAVE·CF3COOH) copolymers. Alternatively, treatment of these random copolymers with methanolic HCl results in the complete hydrolysis of both the Boc-protecting groups as well as the acetate esters, enabling access to well-defined, hydrophilic, polycationic amino acid ester-functionalized poly(vinyl alcohol) materials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...