A facile synthesis of clickable and acid-cleavable PEO for acid-degradable block copolymers†
Abstract
End-functionalized polyethylene oxides containing cleavable
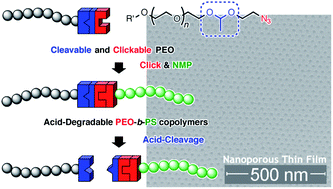
- This article is part of the themed collection: New methods of polymer synthesis

 Please wait while we load your content...
Please wait while we load your content...