Solid-state photochemistry of crystalline pyrazolines: reliable generation and reactivity control of 1,3-biradicals and their potential for the green chemistry synthesis of substituted cyclopropanes
Abstract
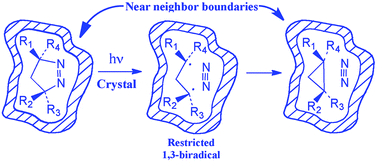
To expand on the limited number of examples that exist in the literature for the solid-state photodenitrogenation of azoalkanes, a series of crystalline 7-alkyl-2,3,7-triazabicyclo[3.3.0]oct-2-ene-6,8-diones with varying 4,4-substituents were prepared. Their photochemical behavior in solution and in the solid state was dependent on the 4,4-substitution of the 1-pyrazoline ring, with unsubstituted pyrazoline 12 giving a mixture of products both in solution and in the solid state.

 Please wait while we load your content...
Please wait while we load your content...