Abstract
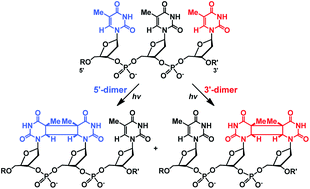
Irradiation of alkane-linked DNA hairpins possessing TTT steps with flanking purine bases yields products identified as the cis–syn (2 + 2) dimers formed between the central thymine and its 3′- and 5′-neighbors. Selective formation of the 3′-dimer is attributed to ground state conformational effects and electron transfer quenching by purine bases.
- This article is part of the themed collection: In honour of Professor Kurt Schaffner

 Please wait while we load your content...
Please wait while we load your content...