Formation of hydrogen peroxide by VUV-photolysis of water and aqueous solutions with methanol†
Abstract
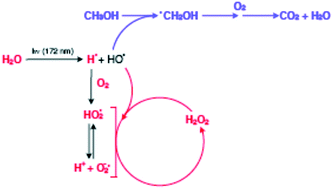
The hydrogen peroxide production upon vacuum ultraviolet (VUV) irradiation of water is reviewed, because published results from the last 10 years lead to conflicting mechanistic interpretations. This work confirms that in pure water, hydrogen peroxide is only produced in the presence of molecular oxygen. Mechanistic schemes explain these findings and confirm earlier statements that recombination of hydroxyl radicals is kinetically disfavoured. In agreement with other recent publications, this work confirms that enhanced hydrogen peroxide production takes place upon VUV irradiation of aqueous solutions of organic compounds. For these investigations, methanol was chosen as an organic model compound. During photolyses, hydrogen peroxide, dissolved molecular oxygen, pH-value of the reaction system, methanol and its products of oxidative degradation were analyzed, and kinetic studies were undertaken to explain the evolution of the concentrations of these components.
- This article is part of the themed collection: In honour of Professor Kurt Schaffner

 Please wait while we load your content...
Please wait while we load your content...