A hypervalent iodine-mediated spirocyclization of 2-(4-hydroxybenzamido)acrylates – unexpected formation of δ-spirolactones†
Abstract
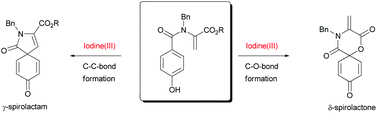
On the way towards a new total synthesis of (S)-arogenate, a novel aryl-λ3-iodane-mediated oxidative spirocyclization of para-substituted

 Please wait while we load your content...
Please wait while we load your content...