Revisiting sesquiterpene biosynthetic pathways leading to santalene and its analogues: a comprehensive mechanistic study†
Abstract
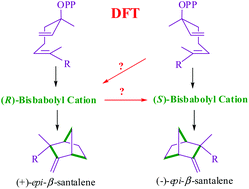
Santalene and bergamotene are the major olefinic sesquiterpenes responsible for the fragrance of sandalwood oil. Herein we report the details of density functional theory investigations on the biosynthetic pathway of this important class of terpenes. The mechanistic study has been found to be effective toward gaining significant new insight into different possibilities for the formation of the key intermediates involved in santalene and bergamotene biosynthesis. The stereoelectronic features of the transition states and intermediates for (i) ring closure of the initial bisabolyl cation, and (ii) skeletal rearrangements in the ensuing bicyclic carbocationic intermediates leading to (−)-epi-β-santalene, (−)-β-santalene, (−)-α-santalene, (+)-epi-β-santalene, exo-β-bergamotene, endo-β-bergamotene, exo-α-bergamotene, and endo-α-bergamotene are presented. Interesting structural features pertaining to certain new carbocationic intermediates (such as b) resulting from the ring closure of bisabolyl cation are discussed. Extensive conformational sampling of all key intermediates along the biosynthetic pathway offered new insight into the role of the isoprenyl side chain conformation in the formation of santalene and its analogues. Although the major bicyclic products in Santalum album appear to arise from the right or left handed helical form of farnesyl pyrophosphate (FPP), different alternatives for their formation are found to be energetically feasible. The interconversion of the exo and endo isomers of bisabolyl cation and a likely epimerization, both with interesting mechanistic implications, are presented. The exo to endo conversion is identified to be energetically more favorable than another pathway emanating from the left handed helical FPP. The role of pyrophosphate (OPP−) in the penultimate deprotonation step leading to olefinic sesquiterpenes is also examined.

 Please wait while we load your content...
Please wait while we load your content...