Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production†
Abstract
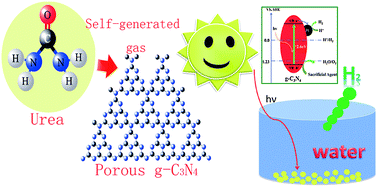
Energy captured directly from sunlight provides an attractive approach towards fulfilling the need for green energy resources on the terawatt scale with minimal environmental impact. Collecting and storing solar energy into
- This article is part of the themed collection: Nanoscale 10th Anniversary: Top Cited Articles

 Please wait while we load your content...
Please wait while we load your content...