Conformational analysis of seven-membered 1-N-iminosugars by NMR and molecular modelling†
Abstract
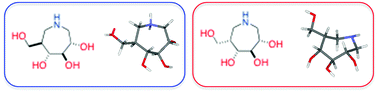
The conformational analysis of a series of tri- and tetrahydroxyazepanes 1–6 designed as noeuromycin mimics has been carried out using
* Corresponding authors
a
Centro de Investigaciones Biológicas, CSIC, 28040 Madrid, Spain
E-mail:
jjbarbero@cib.csic.es
Fax: +34 915360432
Tel: +34 918373112
b UPMC Univ Paris 06, Institut Parisien de Chimie Moléculaire (UMR CNRS 7201), FR 2769, C. 181, 4 place Jussieu, 75005 Paris, France
The conformational analysis of a series of tri- and tetrahydroxyazepanes 1–6 designed as noeuromycin mimics has been carried out using

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
J. Pérez-Castells, M. Fontanella, A. Ardá, F. J. Canãda, M. Sollogoub, Y. Blériot and J. Jiménez-Barbero, New J. Chem., 2012, 36, 1008 DOI: 10.1039/C2NJ20967E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
