Analogues of the HIV-Tat peptide containing Nη-modified arginines as potent inhibitors of protein arginine N-methyltransferases†
Abstract
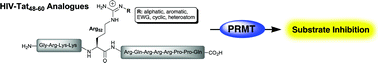
A series of Nη-modified peptides based upon the arginine-rich motif of the HIV-Tat peptide were synthesised and evaluated as substrates and inhibitors of three members of the protein arginine N-methyltransfferase (PRMT) family. PRMT1 and PRMT6 were shown to methylate each of the Tat-peptide analogues tested while PRMT4/CARM1 displayed a lower methylation activity against the same series of peptides. Kinetic assays further revealed that the Tat-peptide analogues also behave as potent substrate-inhibitors of PRMT1 and PRMT6 over specific enzyme concentration ranges. This work provides new insights into the methylating activity and inhibition of PRMTs when interacting with highly positively charged, multiple-arginine-containing peptides.

 Please wait while we load your content...
Please wait while we load your content...