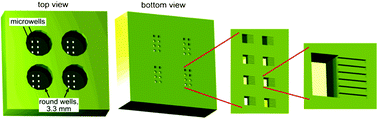
A major obstacle in the treatment of human immunodeficiency virus type 1 (HIV-1) is a sub-population of latently infected CD4+ T lymphocytes. The cellular and viral mechanisms regulating HIV-1 latency are not completely understood, and a promising technique for probing the regulation of HIV-1 latency is single-cell time-lapse microscopy. Unfortunately, CD4+ T lymphocytes rapidly migrate on substrates and spontaneously detach, making them exceedingly difficult to track, hampering single-cell level studies. To overcome these problems, we built microdevices with a three-level architecture. The devices contain arrays of finger-like microchannels to “corral” T-lymphocyte migration, round wells that are accessible to pipetting, and microwells connecting the microchannels with the round wells. T lymphocytes that are loaded into a well first settle into the microwells and then to microchannels by gravity. Within the microchannels, T lymphocytes are in favorable culture conditions because they are in physical contact with each other, under no mechanical stress, and fed from a large reservoir of fresh medium. Most importantly, T lymphocytes in the microchannels are not exposed to any flow and their random migration is restricted to a nearly one-dimensional region, greatly facilitating long-term tracking of multiple cells in time-lapse microscopy. The devices have up to nine separate round wells, making it possible to test up to nine different cell lines or medium conditions in a single experiment. Activated primary CD4+ T lymphocytes, resting primary CD4+ T lymphocytes, and THP-1 monocytic leukemia cells loaded into the devices maintained viability over multiple days. The devices were used to track the fluorescence level of individual primary CD4+ T lymphocytes expressing green fluorescent protein (GFP) for up to 60 hours (h) and to quantify single-cell gene-expression kinetics of four different HIV-1 variants. The kinetics of GFP expression from the lentiviruses in the primary CD4+ T lymphocytes agree with previous measurements of these lentiviral vectors in the immortalized Jurkat T lymphocyte cell line. The proposed devices offer a simple, robust approach to long-term single-cell studies of environmentally sensitive primary lymphocytes.

 Please wait while we load your content...
Please wait while we load your content...