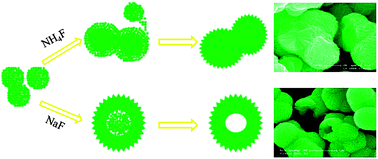
Hierarchical solid and hollow microspheres composed of oriented aligned cone-like SnO2 nanoparticles are prepared by a hydrothermal route using either NH4F or NaF, as morphology controlling agents. Their structures and morphology evolution are comprehensively characterized by TEM, SEM, XRD, XPS and the Brunauer–Emmett–Teller (BET) method, and a formation mechanism is proposed. Both solid and hollow SnO2 microspheres are formed via an Ostwald ripening process undergoing different reorganization paths in the presence of either NH4F or NaF. The solid spheres preferentially recrystallize starting from the cores and grow by consuming adjacent smaller particles, while the hollow spheres preferentially recrystallize starting from outer shells and grow by consuming the entrapped core materials via the mechanism of solid evacuation. As gas sensing materials, both solid and hollow SnO2 microspheres demonstrate sensitive and selective response to several hazardous gases, such as formaldehyde, ammonia, benzene, acetone, and methanol. As lithium storage materials, the hierarchical SnO2 hollow spheres show a higher charge/discharge capacity and better cyclic performance than the hierarchical SnO2 solid spheres. The discharge capacity of the hierarchical SnO2 hollow spheres is 187 mAh g−1 higher than the solid spheres for up to 50 discharge/charge cycles.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...