Laccase-catalyzed oxidation of Hantzsch 1,4-dihydropyridines to pyridines and a new one pot synthesis of pyridines†
Abstract
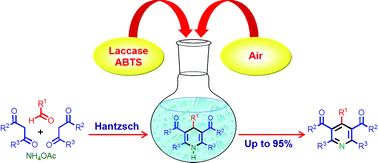
The laccase-catalyzed oxidation of 1,4-dihydropyridines to pyridines using aerial O2 as the oxidant exclusively delivers pyridines with yields up to 95% under mild reaction conditions. Combination of the Hantzsch 1,4-dihydropyridine synthesis with the newly developed laccase-catalyzed oxidation forms the basis of a facile and environmentally benign method for the synthesis of pyridines in one pot.

 Please wait while we load your content...
Please wait while we load your content...