A new electrochemical synthesis route for a BiOI electrode and its conversion to a highly efficient porous BiVO4 photoanode for solar water oxidation†
Abstract
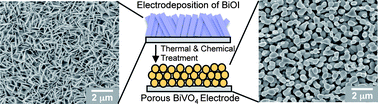
A new electrodeposition condition utilizing p-benzoquinone reduction was developed to produce BiOI electrodes composed of extremely thin 2D BiOI crystals. These electrodes served as precursors to form porous BiVO4 electrodes via mild chemical and thermal treatments. The resulting porous BiVO4 electrodes showed outstanding photoelectrochemical performance for sulfite oxidation reaching 1.25 mA cm−2 at 0.5 V vs. RHE in 0.1 M potassium phosphate buffer (pH 7) containing 0.1 M sodium sulfite. When a ca. 100 nm thick FeOOH layer was deposited on the surface of BiVO4 as an oxygen evolution catalyst, the kinetics of water oxidation was improved to the level of sulfite oxidation and the maximum power point for solar water oxidation was achieved at a bias as low as 0.55 V vs. RHE with a photocurrent density of 1.17 mA cm−2. The remarkable solar water oxidation performance achieved by the porous BiVO4–FeOOH system strongly encourages further morphological and compositional optimizations of the BiVO4-based photoanode systems to realize highly efficient and practical solar water oxidation.

 Please wait while we load your content...
Please wait while we load your content...