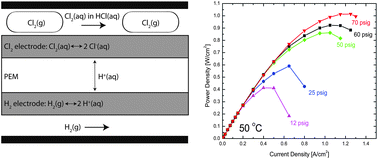
We report the performance of a hydrogen–chlorine electrochemical cell with a chlorine electrode employing a low precious metal content alloy oxide electrocatalyst for the chlorine electrode: (Ru0.09Co0.91)3O4. The cell employs a commercial hydrogen fuel cell electrode and transports protons through a Nafion membrane in both galvanic and electrolytic mode. The peak galvanic power density exceeds 1 W cm−2, which is twice previous literature values. The precious metal loading of the chlorine electrode is below 0.15 mg Ru cm−2. Virtually no activation losses are observed, allowing the cell to run at nearly 0.4 W cm−2 at 90% voltage efficiency. We report the effects of fluid pressure, electrolyte acid concentration, and hydrogen-side humidification on overall cell performance and efficiency. A comparison of our results to the model of Rugolo et al. [Rugolo et al., J. Electrochem. Soc., 2012, 159, B133] points out directions for further performance enhancement. The performance reported here gives these devices promise for applications in carbon sequestration and grid-scale electrical energy storage.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...