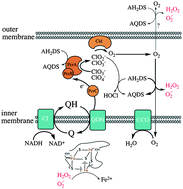
Electrochemical reactors can be used to deliver electrons to bacteria with soluble, redox-active shuttles, and this has been proposed for both biosynthetic and bioremediation purposes. In the case of perchlorate reduction in bioelectrical reactors, very little is known about the mechanism of shuttle oxidation, although this understanding is important for treatment system design and scaleup. In this work, electrochemical, physiological, and proteomic experiments were performed to investigate anthrahydroquinone-2,6-disulfonate (AH2DS) oxidation by Azospira suillum PS under perchlorate reducing conditions. In inoculated bioelectrical reactors with AH2DS as the sole electron donor, only small amounts of cathodic current were observed. However, acetate addition resulted in a rapid increase in cathodic current, concomitant with acetate oxidation. This effect was not seen with nitrate as the terminal electron acceptor or when cells were lysed. We hypothesized that co-utilization of electron donors was a result of normal organotrophic perchlorate reduction, in addition to non-enzymatic reactions between AH2DS and intermediates (chlorite, oxygen) of the pathway. These reactions are expected to result in the production of reactive oxygen species (ROS) and toxicity, which was confirmed in batch growth experiments. Shotgun proteomics revealed significantly increased tandem spectral peptide counts of a diheme c-type cytochrome peroxidase; again, only when perchlorate, acetate, and AH2DS were present. Homologs of this protein relieve oxidative stress by reducing hydrogen peroxide to water. This work highlights the fact that oxidation of inorganic electron donors can occur non-enzymatically, as well as the challenge of targeting a specific metabolism with chemical shuttles that are highly reactive.

 Please wait while we load your content...
Please wait while we load your content...