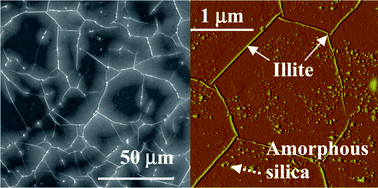
To safely implement geologic carbon sequestration (GCS), a better understanding of geochemical reactions at supercritical CO2 (scCO2)–brine–clay mineral interfaces is necessary. This work investigated phlogopite dissolution and secondary mineral formation after freshly cleaved (001) surfaces were exposed to scCO2–brine systems. Phlogopite was used as a model clay mineral, and scCO2–1 M NaCl–phlogopite systems at 75 °C and 75 atm were chosen to mimic CO2 storage conditions in deep saline aquifers. Additional experiments were also performed at 95 °C to explore the effect of temperature on phlogopite dissolution. The dissolution activation energies for each element were calculated to be 64.2 kJ mol−1 for Si, 53.6 kJ mol−1 for Mg, and 78.4 kJ mol−1 for Al. Over 43 h of reaction time, the activation energy for K dissolution was calculated to be 35.9 kJ mol−1. A whole-mineral activation energy for phlogopite, 62.5 kJ mol−1, was estimated from the weighted mean values of the activation energies of the framework elements (Al, Si, and Mg). Swelling of the phlogopite outer layers, dissolution pit formation, and precipitation of both illite and amorphous silica were dominant at both temperatures. At 75 °C, normalized volumetric surface coverage (μm3/μm2) was 0.34 ± 0.74 for illite and 0.05 ± 0.90 for amorphous silica nanoparticles.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...