Synthesis, structure and band gap energy of covalently linked cluster-assembled materials†
Abstract
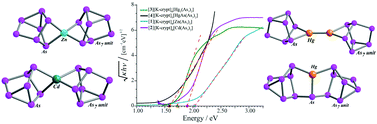
We have synthesized a series of cluster assembled materials in which the building blocks are As73− clusters linked by group 12 metals, Zn, Cd and Hg, to investigate the effect of covalent linkers on the band gap energy. The synthesized assemblies include zero dimensional assemblies of [Zn(As7)2]4−, [Cd(As7)2]4−, [Hg2(As7)2]4−, and [HgAsAs14]3− in which the clusters are separated by cryptated counterions, and assemblies in which [Zn(As7)2]4−, [Cd(As7)2]4− are linked by free alkali atoms into unusual three-dimensional structures. These covalently linked cluster-assembled materials have been characterized by

 Please wait while we load your content...
Please wait while we load your content...