The effect of the number of carbohydrate moieties on the azaphthalocyanine properties†
Abstract
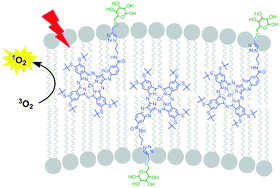
A series of azaphthalocyanines (AzaPc) bearing one, two, four or eight isopropylidene-protected
* Corresponding authors
a
Department of Biophysics and Physical Chemistry, Faculty of Pharmacy in Hradec Kralove, Charles University in Prague, Heyrovskeho 1203, Hradec Kralove, Czech Republic
E-mail:
veronika.novakova@faf.cuni.cz
Fax: +420 495067167
Tel: +420 495067380
b Department of Chemistry, Faculty of Arts and Sciences, Technical University of Istanbul, TR34469 Maslak, Istanbul, Turkey
c
Department of Pharmaceutical Chemistry and Drug Control, Faculty of Pharmacy in Hradec Kralove, Charles University in Prague, Heyrovskeho 1203, Hradec Kralove, Czech Republic
Fax: +420 495067167
Tel: +420 495067257
A series of azaphthalocyanines (AzaPc) bearing one, two, four or eight isopropylidene-protected

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
V. Novakova, R. Z. U. Kobak, R. Kučera, K. Kopecky, M. Miletin, V. Krepsová, J. Ivincová and P. Zimcik, Dalton Trans., 2012, 41, 10596 DOI: 10.1039/C2DT30971H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
