Intramolecular Lewis acid–base pairs based on 4-ethynyl-2,6-lutidine†
Abstract
The reaction of 4-ethynyl-2,6-lutidine, (2,6-Me2)(4-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)C5H2N (2), with B(C6F5)3 afforded the zwitterion [(2,6-Me2)(4-(C6F5)3BC
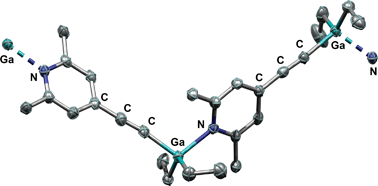
C)C5H2N (2), with B(C6F5)3 afforded the zwitterion [(2,6-Me2)(4-(C6F5)3BC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)C5H2NH] (3) via a deprotonation pathway. By treatment of 2 with the group 13 trialkyls AlMe3, AlEt3, GaMe3, GaEt3 and InMe3, metallation of the ethynyl group afforded compounds 4–8 under extrusion of the corresponding alkane. The resulting products were characterised by elemental analyses and NMR spectroscopy. Compounds 4 and 8 were crystallized from THF and were yielded as monomers with coordinated THF molecules. The gallium compound 7 could be crystallised from benzene and was afforded as coordination polymer. The structures of these three compounds (4·THF, 7 and 8·2THF) were determined by single-crystal X-ray diffraction experiments. The aluminium compounds 4 and 5 show redistribution reaction of their substituents.
C)C5H2NH] (3) via a deprotonation pathway. By treatment of 2 with the group 13 trialkyls AlMe3, AlEt3, GaMe3, GaEt3 and InMe3, metallation of the ethynyl group afforded compounds 4–8 under extrusion of the corresponding alkane. The resulting products were characterised by elemental analyses and NMR spectroscopy. Compounds 4 and 8 were crystallized from THF and were yielded as monomers with coordinated THF molecules. The gallium compound 7 could be crystallised from benzene and was afforded as coordination polymer. The structures of these three compounds (4·THF, 7 and 8·2THF) were determined by single-crystal X-ray diffraction experiments. The aluminium compounds 4 and 5 show redistribution reaction of their substituents.
- This article is part of the themed collection: Frustrated Lewis Pairs

 Please wait while we load your content...
Please wait while we load your content...