A new metallostar complex based on an aluminum(iii) 8-hydroxyquinoline core as a potential bimodal contrast agent†
Abstract
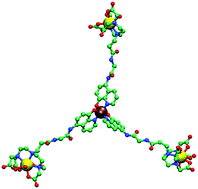
A ditopic DTPA monoamide derivative containing an 8-hydroxyquinoline moiety was synthesized and the corresponding gadolinium(III) complex ([Gd(H5)(H2O)]−) was prepared. After adding aluminum(III), the 8-hydroxyquinoline part self-assembled into a heteropolymetallic triscomplex [(Gd5)3Al(H2O)3]3−. The magnetic and optical properties of this metallostar compound were investigated in order to classify it as a potential in vitro bimodal contrast agent. The

 Please wait while we load your content...
Please wait while we load your content...