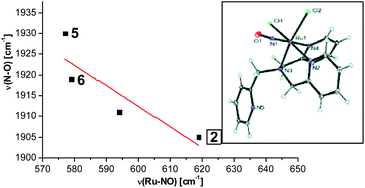
In this paper, the synthesis, structural and spectroscopic characterization of a series of new Ru(III)–nitrosyls of {RuNO}6 type with the coligand TPA (tris(2-pyridylmethyl)amine) are presented. The complex [Ru(TPA)Cl2(NO)]ClO4 (2) was prepared from the Ru(III) precursor [Ru(TPA)Cl2]ClO4 (1) by simple reaction with NO gas. This led to the surprising displacement of one of the pyridine (py) arms of TPA by NO (instead of the substitution of a chloride anion by NO), as confirmed by X-ray crystallography. NO complexes where TPA serves as a tetradentate ligand were obtained by reacting the new Ru(II) precursor [Ru(TPA)(NO2)2] (3) with a strong acid. This leads to the dehydration of nitrite to NO+, and the formation of the {RuNO}6 complex [Ru(TPA)(ONO)(NO)](PF6)2 (4), which was also structurally characterized. Derivatives of 4 where nitrite is replaced by urea (5) or water (6) were also obtained. The nitrosyl complexes obtained this way were then further investigated using IR and FT-Raman spectroscopy. Complex 2 with the two anionic chloride coligands shows the lowest N–O and highest Ru–NO stretching frequencies of 1903 and 619 cm−1 of all the complexes investigated here. Complexes 5 and 6 where TPA serves as a tetradentate ligand show ν(N–O) at higher energy, 1930 and 1917 cm−1, respectively, and ν(Ru–NO) at lower energy, 577 and 579 cm−1, respectively, compared to 2. These vibrational energies, as well as the inverse correlation of ν(N–O) and ν(Ru–NO) observed along this series of complexes, again support the Ru(II)–NO+ type electronic structure previously proposed for {RuNO}6 complexes. Finally, we investigated the photolability of the Ru–NO bond upon irradiation with UV light to determine the quantum yields (ϕ) for NO photorelease in complexes 2, 4, 5, and additional water-soluble complexes [Ru(H2edta)(Cl)(NO)] (7) and [Ru(Hedta)(NO)] (8). Although {RuNO}6 complexes are frequently proposed as NO delivery agents in vivo, studies that investigate how ϕ is affected by the solvent water are lacking. Our results indicate that neutral water is not a solvent that promotes the photodissociation of NO, which would present a major obstacle to the goal of designing {RuNO}6 complexes as photolabile NO delivery agents in vivo.

 Please wait while we load your content...
Please wait while we load your content...