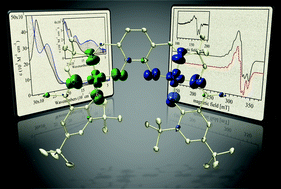
The geometric and electronic structure of a bimetallic Cu Schiff-base complex and its one-electron oxidized form have been investigated. The two salen units in the neutral complex 1 are linked via a bridging catecholate function, and the coupling between the two Cu(II) d9 centres was determined to be weakly antiferromagnetic on the basis of solid-state magnetic studies (J = −3 cm−1), and variable-temperature electron paramagnetic resonance (EPR) (J = −3 cm−1). Theoretical calculations (DFT) were in agreement with the experimental results (J = −7 cm−1), and provided insight into the coupling mechanism for the neutral system. One-electron oxidation provided [1]+ which was observed to have limited stability in solution. The oxidized complex was determined to be a ligand radical species in solution, with the electron hole potentially localized on the redox-active dioxolene, the phenolate ligands, or delocalized over the entire ligand system. Electrochemical experiments and UV-vis-NIR spectroscopy, in combination with density functional theory (DFT) calculations, provided insight into the locus of oxidation and the degree of delocalization in this system. The ligand radical for [1˙]+ was determined experimentally to be localized on the dioxolene bridge with a small amount of spin density on the outer phenolate moieties predicted by the calculations. This assignment was aided via comparison to data for the Ni analogue (Inorg. Chem., 2011, 50, 6746). The resonance Raman spectrum of [1˙]+ (λex = 413 nm) in CH2Cl2 solution clearly exhibited a new band at 1308 cm−1 in comparison to 1, supporting semiquinone formation. Variable-temperature EPR on the three-spin system [1˙]+ did not provide definitive information on the coupling interaction, possibly due to a very small difference in energy between the S = 3/2 and S = 1/2 states and/or a very small zero-field splitting, in combination with significant line-broadening. The data is consistent with a description of the overall electronic structure of [1˙]+ as a bimetallic Cu(II) complex with a bridge-localized semiquinone ligand radical species.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...