Thermodynamic stability, structural and electrical characterization of mixed ionic and electronic conductor La2Mo2O8.96
Abstract
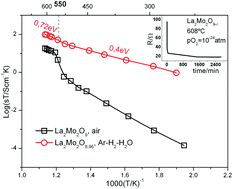
* Corresponding authors
a Instituto Balseiro, Centro Atómico Bariloche, 8400 San Carlos de Bariloche, Río Negro, Argentina
b Consejo Nacional de Investigaciones Científicas y Técnicas CONICET, Argentina
c LUNAM Université, Université du Maine, CNRS UMR 6283, Institut des Molécules et des Matériaux du Mans, Avenue Olivier Messiaen, 72085 Le Mans Cedex 9, France
d
Comisión Nacional de Energía Atómica CNEA, Argentina
E-mail:
caneiro@cab.cnea.gov.ar

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
J. E. Vega-Castillo, U. K. Ravella, G. Corbel, P. Lacorre and A. Caneiro, Dalton Trans., 2012, 41, 7266 DOI: 10.1039/C2DT30261F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
