The significance of the alkene size and the nature of the metal ion in metal–alkene complexes: a theoretical study†
Abstract
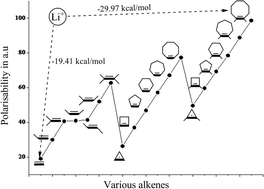
Cation interactions with π-systems are a problem of outstanding contemporary interest and the nature of these interactions seems to be quite different for transition and main group metal ions. In this paper, we have systematically analyzed the contrast in the bonding of Cu+ and main group metal ions. The molecular structures and energetics of the complexes formed by various alkenes (A = CnH2n, n = 2–6; CnH2n − 2, n = 3–8 and CnH2n + 2, n = 5–10) and metal ions (M = Li+, Na+, K+, Ca2+, Mg2+, Cu+ and Zn2+) are investigated by employing ab initio post Hartree–Fock (MP2/6-311++G**) calculations and are reported in the current study. The study, which also aims to evaluate the effect of the size of the alkyl portion attached to the π-system on the complexation energy, indicates a linear relationship between the two. The decreasing order of complexation energy with various metal ion–alkene complexes follows the order Zn2+–A > Mg2+–A > Ca2+–A > Cu+–A > Li+–A > Na+–A > K+–A. The increased charge transfer and the electron density at (3,−1) intermolecular bond critical point corroborates well with the size of the π-system and the complexation energy. The observed deviation from the linear dependency of the Cu+–A complexes is attributed to the dπ → π* back bonding interaction. An energy decomposition analysis via the reduced variational space (RVS) procedure was also carried out to analyze which component among polarization, charge transfer, coulomb and exchange repulsion contributes to the increase in the complexation energy. The RVS results suggest that the polarization component significantly contributes to the increase in the complexation energy when the alkene size increases.

 Please wait while we load your content...
Please wait while we load your content...