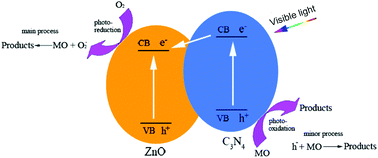
The g-C3N4–ZnO composite photocatalysts with various weight percents of ZnO were synthsized by a simple calcination process. The photocatalysts were characterized by powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HR-TEM), UV-vis diffuse reflection spectroscopy (UV-vis), X-ray photoelectron spectroscopy (XPS) and thermogravimetric analysis (TGA). The PXRD and HR-TEM results show that the composite materials consist of hexagonal wurzite phase ZnO and g-C3N4. The solid-state UV-vis diffuse reflection spectra show that the absorption edge of the composite materials shifts toward the lower energy region and to longer wavelengths in comparison with pure ZnO and g-C3N4. Remarkably, the photocatalytic activity of g-C3N4–ZnO composites has been demonstrated, via photodegradation of Methyl Orange (MO) and p-nitrophenol experiments. The photocatalytic activity of g-C3N4–ZnO for photodegradation of Methyl Orange and p-nitrophenol under visible light irradiation was increased by over 3 and 6 times, respectively, to be much higher than that of single-phase g-C3N4, clearly demonstrating a synergistic effect between ZnO and g-C3N4. The concentrations of Zn2+ in g-C3N4–ZnO system after a photocatalytic reaction at various reaction times were found to be much lower than those for a ZnO system under the same reaction conditions, indicating that the g-C3N4–ZnO composite possesses excellent long-term stability for a photocatalytic reaction in aqueous solutions. Furthermore, a synergistic photocatalysis mechanism between ZnO and g-C3N4 was proposed based on the photodegradation results. Such obviously improved performance of g-C3N4–ZnO can be ascribed mainly to the enhancement of electron–hole separations at the interface of ZnO and g-C3N4.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...