CO assisted N2 functionalization activated by a dinuclear hafnium complex: a DFT mechanistic exploration†
Abstract
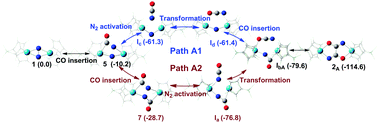
In this paper, the reaction mechanisms of CO assisted N2 cleavage and functionalization activated by a dinuclear hafnium complex are studied using a density function theory (DFT) method. Several key intermediates (Ia, Ib, Ic and Id) with axial/equatorial N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O coordination structures are found to be of importance along reaction pathways of CO assisted N2 functionalization, which could provide a profound theoretical insight into the C–N bond formation and N–N bond cleavage. There are two different attack directions to insert the first CO molecule into the Hf–N bonds of the dinuclear hafnium complex, which lead to C–N bond formation. The calculated results imply that CO insertion into the Hf1–N3 bond (Path A1) reacts more easily than that into the Hf2–N3 bond (Path A3). But for the insertion of the second CO insertion to give 2A, there are two possibilities (Path A1 and Path A2) according to this insertion being after/before N–N bond cleavage. Two pathways (Path A1 and Path A2) are proved to be possible to form final dinitrogen functionalized products (oxamidide 2A, 2B and 2C) in this study, which explain the formation of different oxamidide isomers in CO assisted N2 functionalization activated by a dinuclear hafnium complex.
O coordination structures are found to be of importance along reaction pathways of CO assisted N2 functionalization, which could provide a profound theoretical insight into the C–N bond formation and N–N bond cleavage. There are two different attack directions to insert the first CO molecule into the Hf–N bonds of the dinuclear hafnium complex, which lead to C–N bond formation. The calculated results imply that CO insertion into the Hf1–N3 bond (Path A1) reacts more easily than that into the Hf2–N3 bond (Path A3). But for the insertion of the second CO insertion to give 2A, there are two possibilities (Path A1 and Path A2) according to this insertion being after/before N–N bond cleavage. Two pathways (Path A1 and Path A2) are proved to be possible to form final dinitrogen functionalized products (oxamidide 2A, 2B and 2C) in this study, which explain the formation of different oxamidide isomers in CO assisted N2 functionalization activated by a dinuclear hafnium complex.

 Please wait while we load your content...
Please wait while we load your content...