Gamma-radiolysis-assisted cobalt oxide nanoparticle formation
Abstract
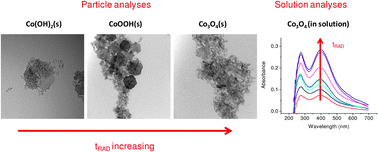
The formation of Co3O4 nano-scale colloid particles by gamma irradiation of CoSO4 solutions was investigated. Solutions of 0.2–0.3 mM CoSO4 at pH 6.0 and 10.6 (air-saturated and Ar-purged) were irradiated at an absorbed dose rate of 5.5 kGy h−1. The resulting concentrations of H2, H2O2, CoII and CoIII species in solution and the chemical composition and sizes of particles that were formed were measured as a function of irradiation time. Particle formation was observed only for initially air-saturated CoSO4 solutions at pH 10.6. Analysis of the particle formation as a function of irradiation time shows that the particles evolve from Co(OH)2 to CoOOH and then to Co3O4. The radiolytic oxidation of CoII to CoIII was completed in 100 min and the chemical composition of the final particles was identified as Co3O4 by XPS, Raman and UV-Vis spectroscopy. Transmission electron microscopy (TEM) images show the final particles are approximately uniform in size, ranging from 8 to 20 nm. A mechanism is proposed to explain the particle formation. A key factor is the low solubility of Co(OH)2 in air-saturated solutions at high pH. This mechanism for particle formation is compared with the mechanism previously reported for the radiolytic formation of γ-FeOOH nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...