Crystal structure of the ionic liquid EtNH3NO3—Insights into the thermal phase behavior of protic ionic liquids†
Abstract
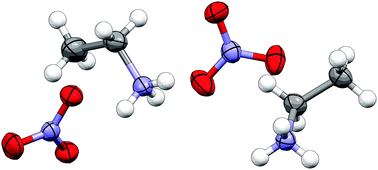
The crystal structure of the salt ethylammonium nitrate (EtNH3NO3) has been determined. EtNH3NO3 is one of the most widely studied protic

 Please wait while we load your content...
Please wait while we load your content...