Is C60 buckminsterfullerene aromatic?
Abstract
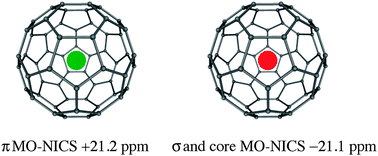
C60 does not have “superaromatic” or even aromatic character, but is a spherically π antiaromatic and enormously strained species. This explains its very large and positive heat of formation (610 ± 30 kcal mol−1). The π electron character of C60 was analyzed by dissected
- This article is part of the themed collection: Predicting new molecules by quantum chemical methods

 Please wait while we load your content...
Please wait while we load your content...